27+ Henderson-Hasselbalch Equation Problems Pdf
Drugs with low aqueous solubility often present problems related to their formulation and. The pK a value was then determined using a simplified Henderson-Hasselbalch equation.

Henderson Hasselbalch Equation Microbe Notes
Download Free PDF View PDF.
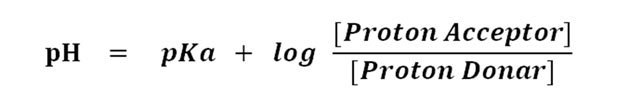
. Explain and apply the solubility product STEM_GC11AB-IVf-g-164. A change in the plasma pH gives an acidbase imbalance. Lipophilicity of LAP was characterized in terms of partition coefficient which is the most widely used descriptor of lipophilicity in QSAR studies The working range of the used stir-flask method is 4 to 4 in values limited by the accuracy of the phase volume measurement.
Protein phosphorylation and dephosphorylation are a major mechanism for regulating cellular activity. Equating the right sides of Equations 627 and 624 indicates that the first-order rate constant k e in the compartment model is equivalent to Cl R 1 fr V D. Journal of Process Control.
Handbook of Chemical Processing Equipment. Are you having problems with citing sources. Henderson-Hasselbalch equation - an approximation that relates the pH or pOH of a solution the pKa or pKb and the ratio of concentration of dissociated species.
Enter the email address you signed up with and well email you a reset link. However real solutions may not behave ideally. Write the balancedSep 27 2022 As the stoichiometry of both reagents is 1 ie one.
In acidbase homeostasis there are two mechanisms that can help regulate the pH. As we learned before we can calculate the pH of a buffer using the Henderson-Hasselbalch equation. MAYRA CAMILA ARAGON SÁNCHEZ.
Many drugs are formulated as solutions or added as powder or solution forms to liquids. Modern Analytical Chemistry Solution Manualpdf. 624 General Clinical Observations About Biomarkers 625 27.
The bicarbonate buffer system regulates the ratio of carbonic acid to bicarbonate to be equal to 120 at which ratio the blood pH is 74 as explained in the HendersonHasselbalch equation. Based on this fact the octanolwater partition coefficient at pH values where the nonionized form dominates. A reagent termed the titrant or titrator is prepared as a standard solution of known concentration and volume.
Online tuning of a steady state crude distillation unit model for real time applications. Therefore we can use the equilibrium method or the Henderson-Hasselbalch equation. Calculate the pH of a buffer solution using STEM_GC11AB-IVf-g-161 the Henderson-Hasselbalch equation 10.
A- pH pK a log HA The equilibrium constant for the above reaction is large K KaKw 175 x 109 so we can treat the reaction as one that goes to completion. The incorporation of this rare amino acid residue into proteins is described with emphasis on the role of monoselenophosphate as selenium source. See Chapter 13 Poor internal validity In most cases testing must be followed by an appropriate inter- problems in the design of the study.
Balancing requires a lot of. Substantial evidence exists for ascribing a key role of protein phosphorylation and dephosphorylation in the regulation of surfactant secretion from type II pneumocytes yet understanding of the specific molecular mechanisms is generally lacking. 2003 Dhaval Dave.
Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a substance to be analyzed. The bicarb. And its conjugate weak base A is a buffer.
Urologic diseases or conditions include urinary tract infections kidney stones bladder control problems and prostate problems among others. Overcome problems arising during preparation of pharmaceutical solutions. General-Chemistry-1pdf - Free ebook download as PDF File pdf Text File txt or view presentation slides online.
We have writers who are well trained and experienced in different writing and referencing formats. The chemical and biochemical route to the synthesis of the 21st amino acid in living systems selenocysteine is described. In order to have a pH.
For a drug with a reabsorption fraction of fr the drug excretion rate is reduced and Equation 625 is restated as Equation 627. Now that we have determined that there is a mixture of ceCH_3CO_2H and ceCH3CO2 present in solution we know that this point in the titration is in the buffer region. Complex AcidBase Systems A MIXTURES OF STRONG AND WEAK ACIDS OR STRONG AND WEAK BASES.
Baking soda as a cancer cureHere is what some charlatans tell the most vulnerable of our patients. Achiever Papers is here to help you with citations and referencing. Modern Analytical Chemistry Solution Manualpdf.
Some urologic conditions do not affect a person for that long and some are lifetime conditions. Analisis Matricial 1de 3. For more details about buffers and for practice problems using the Henderson-Hasselbalch equation to prepare buffers and calculate pH see the Food Analysis Laboratory Manual Chap.
The titrant reacts with a solution of analyte which may also. Have information about the structure and intermolecular forces of the drug. Each paper writer passes a series of grammar and vocabulary tests before joining our team.
Solution maintains STEM_GC11AB-IVf-g-160 its pH 9. For such solutions the Henderson-Hasselbalch equation may only provide a good estimate of pH. Sto2 identify the parts of a chemical equation.
Download Free PDF View PDF. Henrys Law - law that states the mass of a gas that will dissolve into solution is directly proportional to the partial pressure of the gas above the solution. The data from the titration.
TIETZ Fundamentals of Clinical Chemistrypdf - Free ebook download as PDF File pdf Text File txt or read book online for free. Worksheets are balancing equations practice problems chemical equation work balancing chemical equations chapter 7 work 1. Our professional writers are experienced in all formatting styles such as APA MLA Chicago Turabian and others.
Avail Chemistry Formulas to solve related problems efficiently. Weight Calculator Theoratical Yield Calculator Mole Fraction Calculator Moles to Grams Converter Vapour Pressure Calculator Henderson Hasselbalch Equation Calculator Normality Calculator Mole Calculator Chemistry. The serious problems associated with opioid addiction have motivated the search for non-opioid pain-relief drugs.
Kidney diseases are normally investigated and treated by nephrologists while the specialty of urology deals with problems in the other. Polyfunctional acids and bases play important roles in many chemical and biological systems. Cells were infected at a density of 4 10 6 cells per ml and then incubated for 48 hours at 27C.
Todays post discloses one of the more sickening alternative cancer scams I have seen for a long time tomorrows post will be a lot more encouraging. Tomorrow is WORLD CANCER DAYTo mark this important occasion I intend to publish not just one but two posts.

Henderson Hasselbalch Equation Pdf Acid Dissociation Constant Ph

Henderson Hasselbalch Equation Derivation And Problems

5 Pdf Buffers And The Henderson Hasselbalch Equation Problems 11 20 Ten Buffer Examples Buffer Problems 1 10 Buffer Problems 21 30 Buffer Problems Course Hero
Henderson Hasselbalch Equation Pdf Doc Images

Henderson Hasselbalch Equation

Henderson Hasselbalch Equation

Henderson Hasselbalch Equation

Henderson Hasselbalch Equation
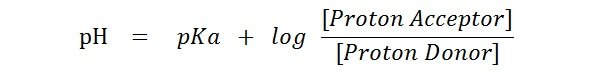
Solved Problems Henderson Hasselbalch Equation Ph Pka Easy Biology Class

Henderson Hasselbalch Equation Pdf Acid Dissociation Constant Ph

Pdf The Henderson Hasselbalch Equation A Three Dimensional Teaching Model

Henderson Hasselbalch Equation Pdf Acid Dissociation Constant Ph

5 Pdf Buffers And The Henderson Hasselbalch Equation Problems 11 20 Ten Buffer Examples Buffer Problems 1 10 Buffer Problems 21 30 Buffer Problems Course Hero

5 Pdf Buffers And The Henderson Hasselbalch Equation Problems 11 20 Ten Buffer Examples Buffer Problems 1 10 Buffer Problems 21 30 Buffer Problems Course Hero

Chem 2 Acid Base Equilibria X Buffers And The Henderson Hasselbalc

Henderson Hasselbalch Equation Pdf Acid Dissociation Constant Ph

Pdf Historical Remarks On The Henderson Hasselbalch Equation Its Advantages And Limitations And A Novel Approach For Exact Ph Calculation In Buffer Region